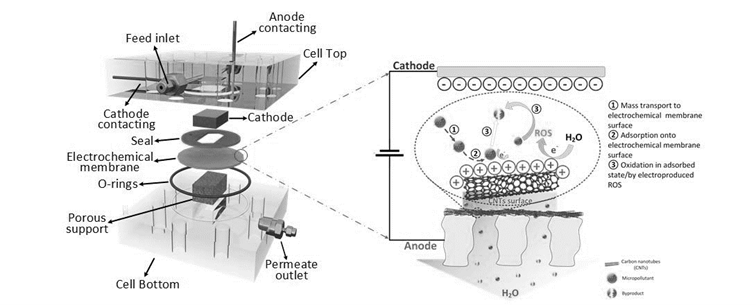
Micropollutants pose serious risks to aquatic wildlife and public health even at trace concentrations (ng/L level). Efficient treatment of the micropollutants in water reuse is crucial in safeguarding the safety of the reclaimed water supply. The designed electrochemical membrane reactor (EMR) with a carbon nanotubes (CNT) membrane that enables simultaneously adsorption, membrane separation and electrochemical reactions is suitable for this. (Project-ID: 02WIL1555)
The severity of water pollution and increasing scarcity of secure drinking water supplies on a global scale have been widely recognized. In 2020, a quarter of population worldwide was estimated to lack access to safely managed drinking water service and nearly a half lack adequate sanitation. In this context, water reuse is paramount in meeting the challenge of severe water deficiencies facing humanity. The removal of micropollutants that pose significant risks to aquatic wildlife and public health at their trace concentrations is crucial for safe water reuse. Complete or even fractional elimination of trace-level micropollutants by conventional municipal wastewater treatment plants is difficult or unattainable. Many of these recalcitrant compounds pass through the treatment process and end up in vital aquatic environment and eventually contaminate surface water, groundwater and even drinking water.
Electrochemical membrane reactor (EMR) has potential to address this challenge. In the EMR process, a conductive membrane serves as the working electrode where the contaminants flows through the membrane pore channels whose electrochemical potential is controlled externally, thus achieving simultaneous electro-adsorption, membrane separation and electrochemical strategies. An EMR with a carbon nanotubes (CNTs) membrane was investigated as part of the NEMWARE project for the treatment of steroid hormone (SH) micropollutant in water at its environmentally relevant concentration (100 ng/L). In particular, the EMR has been able to achieve a > 95% of SH removal at an energy-efficient operating condition (low cell voltage of 2 V, and low pressure of 1 bar at 600 L/m2h.bar). The high efficiency of EMR is attributed to the synergies of the advection-enhanced mass transfer and effects of the electric field under the spatial confinement within nanoscale intraporous structures that contribute to accelerate reaction kinetics.